
Source: cision | Published on: Thursday, 30 May 2024
ZEISS Medical Ecosystem fortifies the CIRRUS 6000 with the U.S. market's largest OCT reference database and advanced cybersecurity features.
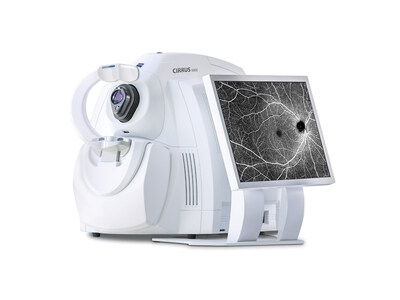
DUBLIN, Calif., May 30, 2024 -- ZEISS Medical Technology announced today that the CIRRUS® 6000 from ZEISS now enables a highly efficient and data-driven workflow for ophthalmologists, supported by the largest OCT (optical coherence tomography) reference database in the U.S. market, as well as newly enhanced cybersecurity features. As part of the ZEISS Medical Ecosystem, the latest OCT device from ZEISS, which recently received clearance by the U.S. Food and Drug Administration (FDA), helps support informed, data-driven patient care decisions across ophthalmic workflows from disease diagnosis to treatment and ongoing management.
Proven Analytics: As the largest reference database across all OCT devices in the U.S. market today, the ZEISS CIRRUS 6000 reference database now offers a comprehensive library of 870 healthy eyes, more than tripling the previous versions, with greater diversity, taking into account different optic disc sizes in addition to age. Elevating the standard of care for patients, the expanded reference database is a useful tool to facilitate individualized approaches to diagnoses and ocular health and disease management.
"ZEISS continues to forge the way toward a future of more personalized care by pioneering state-of-the-art digital workflow innovations that are proven to increase efficiencies and improve patient outcomes," 1 says Euan S. Thomson, Ph.D., President of Ophthalmology Strategic Business Unit and Head of the Digital Business Unit for ZEISS Medical Technology. "Our expansive CIRRUS 6000 reference database provides greater data-driven insights to ophthalmologists."
Performance: The ZEISS CIRRUS 6000 OCT provides high-speed imaging with high-definition detail, a wider field of view, enhanced visualization with AngioPlex® OCT Angiography, and clinically validated applications for retina, glaucoma, and anterior segment – all supporting more insights into a patient's condition in less time with improved security to meet clinical compliance needs.
Patient-First Design: The CIRRUS 6000 offers enhanced security and seamless transfer of patient data from previous generations of CIRRUS for continuity of patient care. Designed to meet evolving clinical compliance and security needs, new cybersecurity features protect against cyber threats, offer advanced data security and instant disaster recovery, provide enhanced password security, enterprise-scale security requirements, and more.
''ZEISS is highly committed to improving patient care and is proud to enhance the CIRRUS OCT brand with digital tools that help keep clinics efficient and compliant. The CIRRUS 6000 enables optimized, data-driven diagnoses and empowers clinicians to manage complex ophthalmic conditions with confidence," says Anuj Kalra, Head of Chronic Disease Management at ZEISS Medical Technology.
Learn more about the enhanced CIRRUS® 6000 from ZEISS at www.zeiss.com/cirrus6000.
1 https://www.dovepress.com/getfile.php?fileID=95911
Contact for investors
Sebastian Frericks
Director Investor Relations
Carl Zeiss Meditec AG
Phone: +49 3641 220 116
Mail: investors.meditec@zeiss.com
Contact for the press
Frank Smith
Head of Global Communications Ophthalmic Devices
Carl Zeiss Meditec Inc.
Phone: +49 3641 220 331
Mail: press.med@zeiss.com
Brief Profile
Carl Zeiss Meditec AG (ISIN: DE0005313704), which is listed on the MDAX and TecDAX of the German stock exchange, is one of the world's leading medical technology companies. The Company supplies innovative technologies and application-oriented solutions designed to help doctors improve the quality of life of their patients. The Company offers complete solutions, including implants and consumables, to diagnose and treat eye diseases. The Company creates innovative visualization solutions in the field of microsurgery. With approximately 4,823 employees worldwide, the Group generated revenue of €2,089.3m in fiscal year 2022/23 (to 30 September).
The Group's head office is located in Jena, Germany, and it has subsidiaries in Germany and abroad; more than 50 percent of its employees are based in the USA, Japan, Spain and France. The Center for Application and Research (CARIn) in Bangalore, India and the Carl Zeiss Innovations Center for Research and Development in Shanghai, China, strengthen the Company's presence in these rapidly developing economies. Around 41 percent of Carl Zeiss Meditec AG's shares are in free float. The remaining approx. 59 percent are held by Carl Zeiss AG, one of the world's leading groups in the optical and optoelectronic industries.
For further information visit: www.zeiss.com/med

Photo - https://mma.prnewswire.com/media/2425218/ZEISS_CIRRUS_6000_OCT.jpg
Logo - https://mma.prnewswire.com/media/546786/ZEISS_v1_Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/zeiss-announces-oct-technology-enhancements-to-better-support-growing-era-of-data-driven-patient-care-302158900.html
View original content:https://www.prnewswire.co.uk/news-releases/zeiss-announces-oct-technology-enhancements-to-better-support-growing-era-of-data-driven-patient-care-302158900.html
